Clickable glutathione using tetrazine-alkene bioorthogonal chemistry for detecting protein glutathionylation†
Abstract
Protein glutathionylation is one of the major cysteine oxidative modifications in response to reactive oxygen species (ROS). We recently developed a clickable glutathione approach for detecting glutathionylation by using a glutathione synthetase mutant (GS M4) that synthesizes azido-glutathione (γGlu-Cys-azido-Ala) in situ in cells. In order to demonstrate the versatility of clickable glutathione and to increase the chemical tools for detecting glutathionylation, we sought to develop clickable glutathione that uses tetrazine-alkene bioorthogonal chemistry. Here we report two alkene-containing glycine surrogates (allyl-Gly and allyl-Ser) for the biosynthesis of clickable glutathione and their use for detection, enrichment, and identification of glutathionylated proteins. Our results provide chemical tools (allyl-Gly and allyl-Ser for GS M4) for versatile characterization of protein glutathionylation. In addition, we show that the active site of GS can be tuned to introduce a small size chemical tag on glutathione for exploring glutathione function in cells.
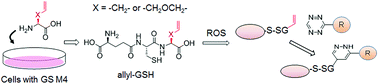

 Please wait while we load your content...
Please wait while we load your content...