Synthesis of crinane utilizing an allylic sulfoxide for the construction of a hydroindole ring via vinylogous C–N bond formation†
Abstract
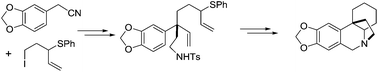
The synthesis of crinane is disclosed via intramolecular C–N bond formation by the displacement of an allylic sulfoxonium salt. The allylic sulfide precursor was synthesized by a ring-closing metathesis reaction. The quaternary carbon stereocenter was created by alkylation of a benzylic cyanide. The allyl sulfide 14 was prepared by adding vinylmagnesium bromide to an α-chlorosulfide.

 Please wait while we load your content...
Please wait while we load your content...