An expeditious synthesis of the ascomycete metabolite rigidiusculamide B†
Abstract
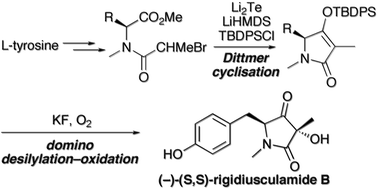
The ascomycete metabolite rigidiusculamide B was synthesised in six steps and 22% overall yield. The key steps were a Li2Te-triggered Dittmer-type Dieckmann cyclisation of an N-(α-haloacyl)tyrosine ester to give a 4-O-silyl tetramate, followed by its fluoride-assisted desilylation–oxidation with oxygen. The product 3-hydroxy-pyrrolidine-2,4-dione was obtained as a 6 : 1 mixture of separable diastereoisomers.

 Please wait while we load your content...
Please wait while we load your content...