Regioselective synthesis of functionalized dihydroisoquinolines from o-alkynylarylaldimines via the Reformatsky reaction†
Abstract
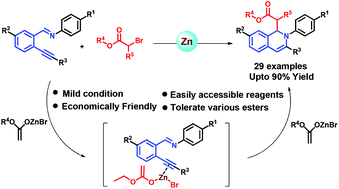
A novel approach for the synthesis of functionalized 1,2-dihydroisoquinolines from o-alkynylarylaldimines via the Reformatsky reaction without the aid of an external Lewis acid has been described. The chemistry involves the dual role of the Reformatsky reagent which has been generated in situ in the reaction. We propose that one molecule of the Reformatsky reagent is being utilised for the activation of alkynes, whereas the second molecule acts as a nucleophile on the iminium carbon. This synthetic pathway has high functional group tolerance, and can be utilized on a gram scale. The proposed mechanistic pathway is well supported by the control experiments. The 1,2-dihydroisoquinoline derivatives showed up to 90% inhibition of the strand transfer activity of the HIV integrase enzyme.

 Please wait while we load your content...
Please wait while we load your content...