Total synthesis of cananginone C and structural revision of debilisone A†
Abstract
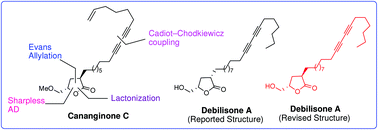
A short, convergent and general strategy for stereoselective total synthesis of biologically active α-substituted γ-hydroxymethyl γ-lactone based natural products cananginone C and debilisone A has been developed. The salient features of this synthesis include Cadiot–Chodkiewicz coupling, Evans allylation, Sharpless asymmetric dihydroxylation and γ-lactonization. The originally proposed structure of debilisone A has been revised.

 Please wait while we load your content...
Please wait while we load your content...