Binaphthyl-based chiral bifunctional organocatalysts for water mediated asymmetric List–Lerner–Barbas aldol reactions†
Abstract
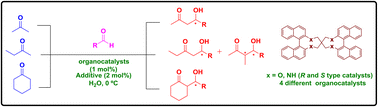
Novel binaphthyl-based chiral bifunctional organocatalysts were designed, synthesized and successfully applied to the asymmetric List–Lerner–Barbas aldol reaction in the presence of water. These organocatalysts were found to be effective catalysts for the reactions of symmetrical, unsymmetrical and cyclic ketones with different aldehydes to give the corresponding aldol products with higher yields (up to 98%) and very good ee's up to 99%. The catalytic system leads to higher yields and selectivities than the previously reported well-known proline based organocatalysts. In addition to the effect of solvent, additives, catalyst concentration, temperature and the substrate scope of the reactions were also investigated.

 Please wait while we load your content...
Please wait while we load your content...