Direct alkylthio-functionalization of unsubstituted perylenediimides†
Abstract
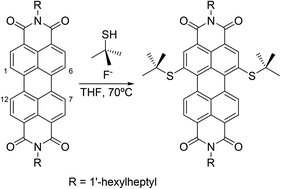
Herein, we report a simple fluoride-mediated reaction for the direct mono- and dialkylthio-functionalization of unsubstituted perylenediimides (PDIs) under very mild conditions. The aromatic substitution reaction offers the possibility to introduce primary, secondary and, even, tertiary alkanethiols either on the 1- or on the 1,6-bay positions of unsubstituted PDIs. 1,6-DialkylthioPDIs show that absorption and fluorescence spectra shifted to the red when compared with the unsubstituted PDI, with Stokes shifts around 70–80 nm.

 Please wait while we load your content...
Please wait while we load your content...