Phosphorylated cyclopropanes in the synthesis of α-alkylidene-γ-butyrolactones: total synthesis of (±)-savinin, (±)-gadain and (±)-peperomin E†
Abstract
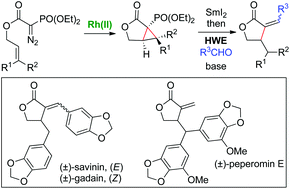
Phosphorylated cyclopropanes, generated via the Rh(II)-catalysed intramolecular cyclopropanation of α-(diethoxyphosphoryl)acetates, have been found to be useful precursors in the synthesis of α-alkylidene-γ-butyrolactones. These cyclopropyl intermediates undergo regioselective reductive ring-opening and subsequent Horner–Wadsworth–Emmons olefination to complete the synthesis. Total syntheses of (±)-savinin and (±)-gadain, as well as the first total synthesis of (±)-peperomin E, are all described using this method.
- This article is part of the themed collection: Celebrating our 2018 prize and award winners

 Please wait while we load your content...
Please wait while we load your content...