The synthesis of a pyridine-N-oxide isophthalamide rotaxane utilizing supplementary amide hydrogen bond interactions†
Abstract
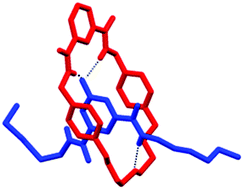
The synthesis of a pyridine-N-oxide containing rotaxane, not requiring an additional ionic template, has been achieved in 32% yield. Successful rotaxane formation is dependent upon the structure of the isophthalamide macrocycle used, an observation which has been rationalised by a combination of NMR spectroscopy, X-ray crystallography and computational modelling.

 Please wait while we load your content...
Please wait while we load your content...