General synthesis of P-stereogenic compounds: the menthyl phosphinate approach†
Abstract
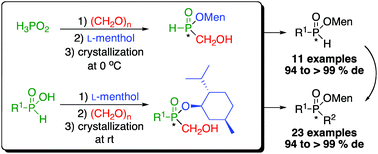
Easily prepared menthyl phosphinates of high diastereoisomeric purity provide versatile intermediates for the synthesis of P-stereogenic compounds. Previous efforts starting about fifty years ago have been hampered by a lack of generality so the menthyl route has been nearly abandoned. Herein we provide a general solution to this long-standing problem and describe a general synthesis of menthyl H-phosphinate and disubstituted phosphinate esters. The method to prepare these versatile precursors relies on a simple and inexpensive process avoiding the use of phosphorus trichloride, Grignard reagents, and complicated cryogenic crystallizations. Established protocols can then be employed to synthesize P-stereogenic secondary and tertiary phosphine oxides and therefore P-stereogenic phosphine ligands.

 Please wait while we load your content...
Please wait while we load your content...