Synthesis of the 2-formylpyrrole spiroketal pollenopyrroside A and structural elucidation of xylapyrroside A, shensongine A and capparisine B†
Abstract
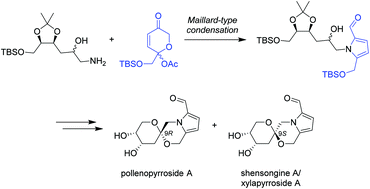
A convergent synthesis of the 2-formyl pyrrole spiroketal pollenopyrroside A is reported. The key step involves a Maillard-type condensation of an amine derived from deoxy-D-ribose with a dihydropyranone to furnish the 2-formylpyrrole ring system. Spectroscopic and physical data of 9-epi-pollenopyrroside A are also provided, elucidating the structures of the previously isolated 2-formylpyrrole spiroketals capparisine B, shensongine A and xylapyrroside A.

 Please wait while we load your content...
Please wait while we load your content...