Enantioselective synthesis of 2,3-disubstituted trans-2,3-dihydrobenzofurans using a Brønsted base/thiourea bifunctional catalyst†
Abstract
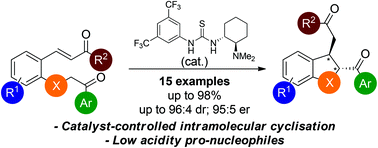
The diastereo- and enantioselective synthesis of 2,3-disubstituted trans-2,3-dihydrobenzofuran derivatives (15 examples, up to 96 : 4 dr, 95 : 5 er) via intramolecular Michael addition has been developed using keto–enone substrates and a bifunctional tertiary amine–thiourea catalyst. This methodology was extended to include non-activated ketone pro-nucleophiles for the synthesis of 2,3-disubstituted indane and 3,4-disubstituted tetrahydrofuran derivatives.

 Please wait while we load your content...
Please wait while we load your content...