A mechanistic study on the inhibition of α-chymotrypsin by a macrocyclic peptidomimetic aldehyde†
Abstract
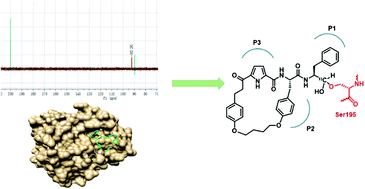
Here we describe an NMR and X-ray crystallography-based characterisation of the mechanism by which a new class of macrocyclic peptidomimetic aldehyde inhibits α-chymotrypsin. In particular, a 13C-labelled analogue of the inhibitor was prepared and used in NMR experiments to confirm formation of a hemiacetal intermediate on binding with α-chymotrypsin. Analysis of an X-ray crystallographic structure in complex with α-chymotrypsin reveals that the backbone adopts a stable β-strand conformation as per its design. Binding is further stabilised by interaction with the oxyanion hole near the S1 subsite and multiple hydrogen bonds.
- This article is part of the themed collection: Macrocycles with bio-related applications

 Please wait while we load your content...
Please wait while we load your content...