Asymmetric synthesis of (+)-17-epi-methoxy-kauran-3-one through tandem oxidative polycyclization-pinacol process†
Abstract
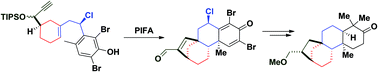
A synthesis of (+)-17-epi-methoxy-kauran-3-one, an O-methylated isomer of the natural diterpene 17-hydroxy-kauran-3-one, has been achieved. The strategy is based on a diastereoselective oxidative polycyclization-pinacol tandem process consisting of transforming a functionalized phenol into a compact and complex tetracycle, which represents the main core of kaurane family members. The synthesis also includes an enantioselective Yamamoto's allylation, a diastereoselective Ru-catalyzed hydrocyanation, a ring-closing metathesis and a reductive isomerization process as key steps. The structure of our synthetic substrate was determined through comparison with an O-methylated derivative of the natural compound.

 Please wait while we load your content...
Please wait while we load your content...