Synthesis and immunological evaluation of MUC1 glycopeptide conjugates bearing N-acetyl modified STn derivatives as anticancer vaccines†
Abstract
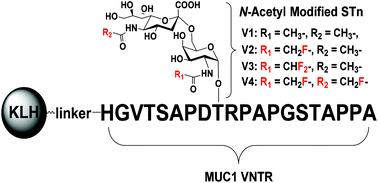
Glycoprotein MUC1 is an attractive target for anti-tumor vaccine development. However, the weak immunogenicity of MUC1 remains a significant problem. To solve this problem, several STn derivatives with N-acetyl modifications were synthesized and incorporated into a 20-amino acid MUC1 tandem repeat sequence. The modified STn-MUC1 glycopeptides were further connected to a carrier protein keyhole limpet hemocyanin (KLH). The immunological effects of these synthetic vaccine conjugates were evaluated using the BALB/c mouse model. The results showed that vaccine V2 elicited higher titers of antibodies which cross-reacted with the native STn-MUC1 antigen. Moreover, the elicited antisera reacted with the STn-MUC1 antigen-positive tumor cells, indicating that the carbohydrate antigen modification strategy may hold potential to overcome the weak immunogenicity of natural MUC1 glycopeptides.

 Please wait while we load your content...
Please wait while we load your content...