6-Bromo-7-hydroxy-3-methylcoumarin (mBhc) is an efficient multi-photon labile protecting group for thiol caging and three-dimensional chemical patterning†
Abstract
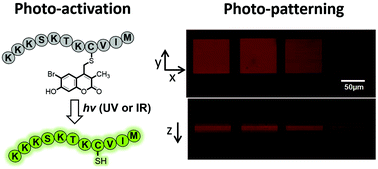
The photochemical release of chemical reagents and bioactive molecules provides a useful tool for spatio-temporal control of biological processes. However, achieving this goal requires the development of highly efficient one- and two-photon sensitive photo-cleavable protecting groups. Thiol-containing compounds play critical roles in biological systems and bioengineering applications. While potentially useful for sulfhydryl protection, the 6-bromo-7-hydroxy coumarin-4-ylmethyl (Bhc) group can undergo an undesired photoisomerization reaction upon irradiation that limits its uncaging efficiency. To address this issue, here we describe the development of 6-bromo-7-hydroxy-3-methylcoumarin-4-ylmethyl (mBhc) as an improved group for thiol-protection. One- and two-photon photolysis reactions demonstrate that a peptide containing a mBhc-caged thiol undergoes clean and efficient photo-cleavage upon irradiation without detectable photoisomer production. To test its utility for biological studies, a K-Ras-derived peptide containing an mBhc-protected thiol was prepared by solid phase peptide synthesis using Fmoc-Cys(mBhc)-OH for the introduction of the caged thiol. Irradiation of that peptide using either UV or near IR light in presence of protein farnesyltransferase (PFTase), resulted in generation of the free peptide which was then recognized by the enzyme and became farnesylated. To show the utility of this caging group in biomaterial applications, we covalently modified hydrogels with mBhc-protected cysteamine. Using multi-photon confocal microscopy, highly defined volumes of free thiols were generated inside the hydrogels and visualized via reaction with a sulfhydryl-reactive fluorophore. The simple synthesis of mBhc and its efficient removal by one- and two-photon processes make it an attractive protecting group for thiol caging in a variety of applications.
- This article is part of the themed collection: Selective Chemistry with Peptides and Proteins

 Please wait while we load your content...
Please wait while we load your content...