Biosynthesis of isoxazolin-5-one and 3-nitropropanoic acid containing glucosides in juvenile Chrysomelina†
Abstract
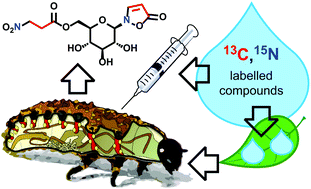
Stable-isotope-labeled precursors were used to establish the biosynthetic pathway leading from β-alanine towards isoxazolin-5-one glucoside 1 and its 3-nitropropanoate (3-NPA) ester 2 in Chrysomelina larvae. Both structural elements originate from sequestered plant-derived β-alanine or from propanoyl-CoA that is derived from the degradation of some essential amino acids, e.g. valine. β-Alanine is converted into 3-NPA and isoxazolinone 5 by consecutive oxidations of the amino group of β-Ala. Substituting the diphospho group of α-UDP-glucose with 5 generates the isoxazolin-5-one glucoside 1, which serves in the circulating hemolymph of the larva as a platform for esterification with 3-nitropropanoyl-CoA. The pathway was validated with larvae of Phaedon cochleariae, Chrysomela populi as well as Gastrophysa viridula.
 Please wait while we load your content...
Please wait while we load your content...