Synthesis and amylin receptor activity of glycomimetics of pramlintide using click chemistry†
Abstract
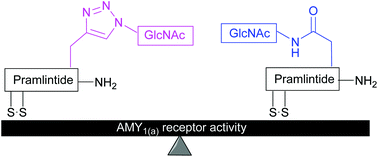
Pramlintide (Symlin®), a synthetic analogue of the neuroendocrine hormone amylin, is devoid of the tendency to form cytotoxic amyloid fibrils and is currently used in patients with type I and type II diabetes mellitus as an adjunctive therapy with insulin or insulin analogues. As part of an on-going search for a pramlintide analogue with improved pharmacokinetic properties, we herein report the synthesis of mono- and di-glycosylated analogues of pramlintide and their activity at the AMY1(a) receptor. Introduction of N-glycosylated amino acids into the pramlintide sequence afforded the native N-linked glycomimetics whilst use of Cu(I)-catalysed azide–alkyne 1,3-dipolar cycloaddition (click) chemistry delivered 1,2,3-triazole linked glycomimetics. AMY1(a) receptor activity was retained by incorporation of single or multiple GlcNAc moieties at positions 21 and 35 of native pramlintide. Importantly, no difference in AMY1(a) activity was observed between native N-linked glycomimetics and 1,2,3-triazole linked glycomimetics demonstrating that the click variants can act as surrogates for the native N-glycosides in a biological setting.
- This article is part of the themed collection: Selective Chemistry with Peptides and Proteins

 Please wait while we load your content...
Please wait while we load your content...