Directing activator-assisted regio- and oxidation state-selective aerobic oxidation of secondary C(sp3)–H bonds in aliphatic alcohols†
Abstract
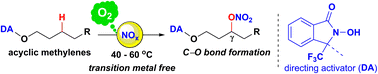
The regioselective conversion of an unactivated C(sp3)–H bond of a methylene carbon (CH2) into a C–O single bond is an attractive reaction in organic synthesis. Herein, we present a strategy for a regio- and oxidation state-selective aerobic C–H oxidation based on an N-hydroxyamide-derived directing activator (DA), which is attached to a hydroxy group in alcohol substrates. The DA reacts with NOx species generated in situ from NaNO2, a Brønsted acid, and aerobic oxygen, and effectively generates an amidoxyl radical from the N-hydroxy moiety of the DA. Then, the amidoxyl radical promotes site-selective intramolecular C–H abstraction from methylenes with γ- (or δ-) selectivity. The thus-generated methylene radicals are trapped by molecular oxygen and NO. This process results in the predominant formation of nitrate esters as products, which suppresses undesired overoxidation. The products can be easily converted into alcohols after hydrogenolysis.

 Please wait while we load your content...
Please wait while we load your content...