Optimization of fluorescent 8-heteroaryl-guanine probes for monitoring protein-mediated duplex → G-quadruplex exchange†
Abstract
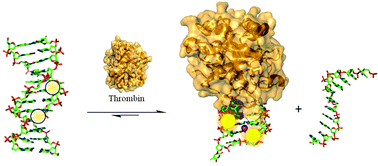
In this study, we describe the thermal and optical properties of the thrombin binding aptamer (TBA) that has been modified at syn-G-tetrad positions with fluorescent 8-heteroaryl-2′-deoxyguanosine derivatives consisting of pyrrolyl (PyrdG), furyl (FurdG), thienyl (ThdG), benzofuryl (BfurdG), indolyl (InddG) and benzothienyl (BthdG). Insertion of the modified base into the syn-G5 position of TBA decreases duplex stability, but enhances stability of the antiparallel G-quadruplex (GQ) structure produced by TBA in the presence of K+ ion and its molecular target, thrombin. The resulting modified TBA (mTBA) oligonucleotides have been employed in duplex → GQ exchange to monitor thrombin binding affinity and rates of GQ formation driven by thrombin binding. Our studies demonstrate that 8-heteroaryl-dG bases can be inserted into syn-G-tetrad positions of TBA without perturbing thrombin binding affinity and that the 8-thienyl-dG (ThdG) analog is particularly useful as an emissive probe for monitoring duplex → GQ exchange due to its heightened emissive sensitivity to change in DNA topology compared to the other 8-heteroaryl-dG analogs. The positional impact of a single ThdG probe versus multiple ThdG incorporation at syn-G sites of TBA highlight an advantage for di-substituted mTBA oligonucleotides for increased emission intensity and rates of duplex → GQ exchange that can be vital for diagnostics through aptamer detection strategies.

 Please wait while we load your content...
Please wait while we load your content...