A combined computational and experimental investigation of the oxidative ring-opening of cyclic ethers by oxoammonium cations†
Abstract
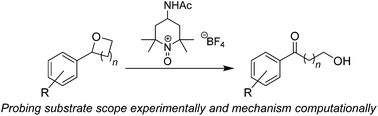
The propensity of oxoammonium cations to facilitate the oxidative ring-opening of cyclic ethers to their corresponding distal hydroxy ketones is investigated. The reaction has been evaluated using experimental and computational methods to gain deeper insight into trends in reactivity.

 Please wait while we load your content...
Please wait while we load your content...