Synthesis and evaluation of a desymmetrised synthetic lectin: an approach to carbohydrate receptors with improved versatility†
Abstract
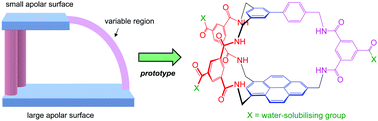
A new synthetic lectin features apolar surfaces provided by two different aromatic components (biphenyl and pyrenyl). Affinities up to 260 M−1 are recorded for carbohydrates in water. The desymmetrised design has potential for variation to give receptors with a broadened range of capabilities.

 Please wait while we load your content...
Please wait while we load your content...