Pushing the limits of catalytic C–H amination in polyoxygenated cyclobutanes†
Abstract
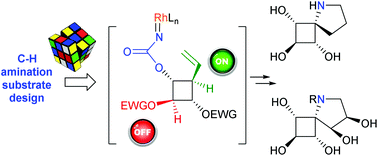
A synthetic route to a new class of conformationally constrained iminosugars based on a 5-azaspiro[3.4]octane skeleton has been developed by way of Rh(II)-catalyzed C(sp3)–H amination. The pivotal stereocontrolled formation of the quaternary C–N bond by insertion into the C–H bonds of the cyclobutane ring was explored with a series of polyoxygenated substrates. In addition to anticipated regioselective issues induced by the high density of activated α-ethereal C–H bonds, this systematic study showed that cyclobutane C–H bonds were, in general, poorly reactive towards catalytic C–H amination. This was demonstrated inter alia by the unexpected formation of a oxathiazonane derivative, which constitutes a very rare example of the formation of a 9-membered ring by way of catalyzed C(sp3)–H amination. A complete stereocontrol could be however achieved by activating the key insertion position as an allylic C–H bond in combination with reducing the electron density at the undesired C–H insertion sites by using electron-withdrawing protecting groups. Preliminary biological evaluations of the synthesized spiro-iminosugars were performed, which led to the identification of a new class of correctors of the defective F508del-CFTR gating involved in cystic fibrosis.

 Please wait while we load your content...
Please wait while we load your content...