The total synthesis of calcium atorvastatin†
Abstract
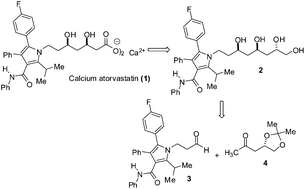
A practical and convergent asymmetric route to calcium atorvastatin (1) is reported. The synthesis of calcium atorvastatin (1) was performed using the remote 1,5-anti asymmetric induction in the boron-mediated aldol reaction of β-alkoxy methylketone (4) with pyrrolic aldehyde (3) as a key step. Calcium atorvastatin was obtained from aldehyde (3) after 6 steps, with a 41% overall yield.

 Please wait while we load your content...
Please wait while we load your content...