Exploring the role of the α-carboxyphosphonate moiety in the HIV-RT activity of α-carboxy nucleoside phosphonates†
Abstract
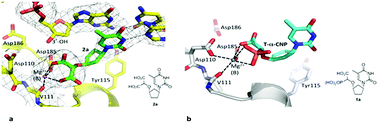
As α-carboxy nucleoside phosphonates (α-CNPs) have demonstrated a novel mode of action of HIV-1 reverse transcriptase inhibition, structurally related derivatives were synthesized, namely the malonate 2, the unsaturated and saturated bisphosphonates 3 and 4, respectively and the amide 5. These compounds were evaluated for inhibition of HIV-1 reverse transcriptase in cell-free assays. The importance of the α-carboxy phosphonoacetic acid moiety for achieving reverse transcriptase inhibition, without the need for prior phosphorylation, was confirmed. The malonate derivative 2 was less active by two orders of magnitude than the original α-CNPs, while displaying the same pattern of kinetic behavior; interestingly the activity resides in the “L”-enantiomer of 2, as seen with the earlier series of α-CNPs. A crystal structure with an RT/DNA complex at 2.95 Å resolution revealed the binding of the “L”-enantiomer of 2, at the polymerase active site with a weaker metal ion chelation environment compared to 1a (T-α-CNP) which may explain the lower inhibitory activity of 2.

 Please wait while we load your content...
Please wait while we load your content...