Catalytic asymmetric hetero-Diels–Alder reactions of enones with isatins to access functionalized spirooxindole tetrahydropyrans: scope, derivatization, and discovery of bioactives†
Abstract
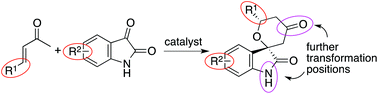
The development of concise methods for the synthesis of functionalized small molecules is important in the search for new bioactive molecules. To contribute to this, we have developed oxa-hetero-Diels–Alder reactions of enones with isatins catalyzed by amine-based catalyst systems. Various spirooxindole tetrahydropyranones were synthesized either in enantiomerically enriched forms or as racemic forms depending on the catalyst system. The reaction products were further transformed at the ketone carbonyl group and the indole nitrogen. Using these reactions, functionalized spirooxindole tetrahydropyran derivatives with functional groups in four directions in a three-dimensional space were concisely obtained. From these synthesized compounds, an inhibitor of human ion channel Nav1.7 with μM-level activity was identified, indicating that the developed reaction methods are useful for providing molecules for the discovery of new biofunctional molecules.

 Please wait while we load your content...
Please wait while we load your content...