Synthesis of tetra-substituted 5-trifluoromethylpyrazoles via sequential halogenation/palladium-catalyzed C–C and C–N cross-coupling†
Abstract
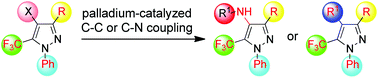
A mild and efficient protocol for the assembly of tetra-substituted 5-trifluoromethylpyrazoles is presented, involving halogenation at the 4-position of readily prepared tri-substituted 5-trifluoromethylpyrazoles to give 4-halo-1-phenyl-5-trifluoromethyl pyrazoles, and subsequent palladium-catalyzed Negishi or Buchwald–Hartwig cross-couplings to install carbon or nitrogen-based 4-substituents. Key to the success of these challenging cross-couplings is the use of XPhos and JosiPhos CyPF-tBu ligands, respectively.

 Please wait while we load your content...
Please wait while we load your content...