Synthesis of functionalized 5-substituted thiazolidine-2-thiones via adscititious xanthate-promoted radical cyclization of allyl(alkyl/aryl)dithiocarbamates†
Abstract
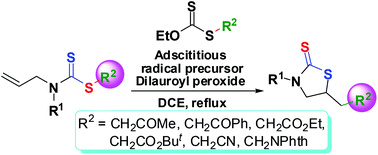
Functionalized 5-substituted thiazolidine-2-thiones were synthesized efficiently from alkyl allyl(alkyl/aryl)-dithiocarbamates via radical cyclization with the corresponding S-alkyl O-ethyl xanthates as the adscititious radical precursors. The application of the adscititious radical precursors improves not only the yields, but also the efficiency in the radical cyclization reaction significantly. The current adscititious radical precursor method provides a new strategy for the achievement and improvement of some radical reactions which are hardly or difficultly realized by the traditional direct methods.
 Please wait while we load your content...
Please wait while we load your content...