Docking study and biological evaluation of pyrrolidine-based iminosugars as pharmacological chaperones for Gaucher disease†
Abstract
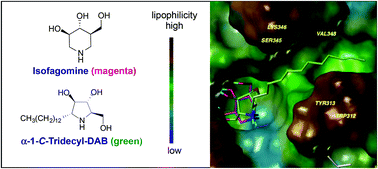
We report on the synthesis and biological evaluation of a series of α-1-C-alkylated 1,4-dideoxy-1,4-imino-D-arabinitol (DAB) derivatives as pharmacological chaperones for Gaucher disease. The parent compound, DAB, did not show inhibition of human β-glucocerebrosidase but showed moderate intestinal α-glucosidase inhibition; in contrast, extension of α-1-C-alkyl chain length gave a series of highly potent and selective inhibitors of the β-glucocerebrosidase. Our design of α-1-C-tridecyl-DAB (5j) produced a potent inhibitor of the β-glucocerebrosidase, with IC50 value of 0.77 μM. A molecular docking study revealed that the α-1-C-tridecyl group has a favorable interaction with the hydrophobic pocket and the sugar analogue part (DAB) interacted with essential hydrogen bonds formed to Asp127, Glu235 and Glu340. Furthermore, α-1-C-tridecyl-DAB (5j) displayed enhancement of activity at an effective concentration 10-times lower than isofagomine. α-1-C-Tridecyl-DAB therefore provides the first example of a pyrrolidine iminosugar as a new class of promising pharmacological chaperones with the potential for treatment of Gaucher disease.

 Please wait while we load your content...
Please wait while we load your content...