Design of a fluorescent ligand targeting the S-adenosylmethionine binding site of the histone methyltransferase MLL1†
Abstract
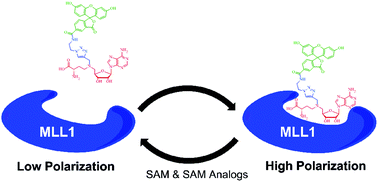
The histone methyltransferase MLL1 has been linked to translocation-associated gene fusion in childhood leukemias and is an attractive drug target. High-throughput biochemical analysis of MLL1 methyltransferase activity requires the production of at least a trimeric complex of MLL1, RbBP5 and WDR5 to elicit robust activity. Production of trimeric and higher order MLL1 complexes in the quantities and reproducibility required for high-throughput screening presents a significant impediment to MLL1 drug discovery efforts. We present here a small molecule fluorescent ligand (FL-NAH, 6) that is able to bind to the S-adenosylmethionine (SAM) binding site of MLL1 in a manner independent of the associated complex members. We have used FL-NAH to develop a fluorescence polarization-based SAM displacement assay in a 384-well format targeting the MLL1 SET domain in the absence of associated complex members. FL-NAH competes with SAM and is displaced from the MLL1 SET domain by other SAM-binding site ligands with Kdisp values similar to the higher-order complexes, but is unaffected by the H3 peptide substrate. This assay enables screening for SAM-competitive MLL1 inhibitors without requiring the use of trimeric or higher order MLL1 complexes, significantly reducing screening time and cost.
- This article is part of the themed collection: 7th EuCheMS Chemistry Congress – Molecular frontiers and global challenges

 Please wait while we load your content...
Please wait while we load your content...