Homogeneous cationic substitution for two-dimensional layered metal oxide nanosheets via a galvanic exchange reaction†
Abstract
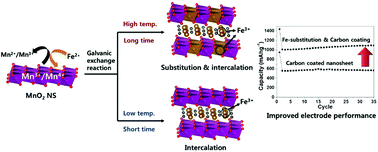
The galvanic exchange reaction of an exfoliated 2D layered metal oxide nanosheet (NS) with excess substituent metal cations enables the synthesis of a mixed metal oxide 2D NS with controllable cation compositions and physicochemical properties. The reaction of the exfoliated MnO2 NS with Fe2+ or Sn2+ ions at 90 °C induces the uniform galvanic replacement of Mn ions with these substituent ions, whereas the same reaction at 25 °C results in the intercalative restacking of the negatively-charged MnO2 NS with Fe2+ or Sn2+ cations. Upon the galvanic exchange reaction, the highly anisotropic MnO2 2D NS retains its original 2D morphology and layered structure, which is in stark contrast to 0D nanoparticles yielding hollow nanospheres via the galvanic exchange reaction. This observation is attributable to the thin thickness of the 2D NS allowing the simultaneous replacement of all the component surface-exposed metal ions. The resulting substitution of the MnO2 NS with Fe and Sn ions remarkably improves the electrode performance of the carbon-coated derivatives of the MnO2 NS for lithium ion batteries. The present study clearly demonstrates that the galvanic exchange reaction can provide an efficient method not only to tailor cation compositions but also to improve the functionalities of 2D metal oxide NSs and their carbon-coated derivatives.


 Please wait while we load your content...
Please wait while we load your content...