Phase crossover in transition metal dichalcogenide nanoclusters†
Abstract
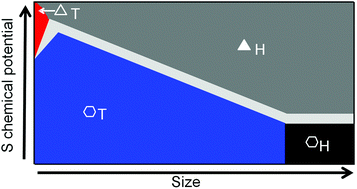
We perform comprehensive first-principles analyses on the stability of MX2 nanoclusters. The MX2 (M = Mo, W; X = S) clusters thermodynamically show a high level of phase variability, i.e. varying from the H phase, which is the ground state of two-dimensional MX2, to the T phase with the decreasing cluster size or chemical potential of X. In addition, the lower chemical potential of X endows the clusters with a stronger propensity of shaping in hexagons, instead of commonly observed triangles, consistent with recent experiments. Based on numerical analyses, we further express the energy of different types of clusters in terms of chemical potential and cluster size, and map out a structural phase diagram. These findings call for a revisit of the lattice structures of MX2 clusters and may also rationalize the frequent observation of meta-stable T domains embedded in otherwise perfect H-MX2 monolayers.

 Please wait while we load your content...
Please wait while we load your content...