A high performance flexible all solid state supercapacitor based on the MnO2 sphere coated macro/mesoporous Ni/C electrode and ionic conducting electrolyte†
Abstract
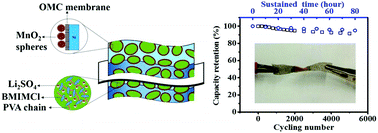
A high contact resistance between the active materials and the current collector, a low ionic conductivity of the gel electrolyte, and an impenetrable electrode structure are the three major barriers which greatly limit the capacitance of MnO2 in solid state supercapacitors. As a potential solution to these problems, in this work we report a novel electrode for solid state supercapacitors, based on a ternary system composed of hierarchical MnO2 spheres as the active material, macroporous Ni foam as gel penetrable skeletons and an ordered mesoporous carbon (OMC) membrane as the charge-transport accelerating layer. By employing butyl-3-methylimidazolium chloride (BMIMCl) modified gels as the ionic conducting electrolyte, the utilization efficiency of MnO2 on the specific capacitance was enhanced up to 88% of the theoretical value, delivering a volumetric capacitance of 81 F cm−3, which is the highest value among MnO2 based solid state supercapacitors. Moreover, such a flexible device exhibits exceptional volumetric energy and power density (6.6 Wh L−1 and 549 W L−1, based on the whole device volume) combined with a small capacity loss of 8.5% after 6000 cycles under twisting. These encouraging findings unambiguously overcome the energy bottleneck of MnO2 in solid state supercapacitors, and open up a new application of macro/mesoporous materials in flexible devices.


 Please wait while we load your content...
Please wait while we load your content...