Electron beam controlled covalent attachment of small organic molecules to graphene†
Abstract
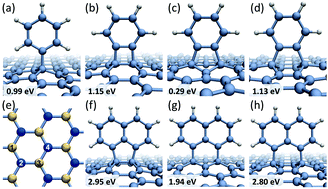
The electron beam induced functionalization of graphene through the formation of covalent bonds between free radicals of polyaromatic molecules and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds of pristine graphene surface has been explored using first principles calculations and high-resolution transmission electron microscopy. We show that the energetically strongest attachment of the radicals occurs along the armchair direction in graphene to carbon atoms residing in different graphene sub-lattices. The radicals tend to assume vertical position on graphene substrate irrespective of direction of the bonding and the initial configuration. The “standing up” molecules, covalently anchored to graphene, exhibit two types of oscillatory motion – bending and twisting – caused by the presence of acoustic phonons in graphene and dispersion attraction to the substrate. The theoretically derived mechanisms are confirmed by near atomic resolution imaging of individual perchlorocoronene (C24Cl12) molecules on graphene. Our results facilitate the understanding of controlled functionalization of graphene employing electron irradiation as well as mechanisms of attachment of impurities via the processing of graphene nanoelectronic devices by electron beam lithography.
C bonds of pristine graphene surface has been explored using first principles calculations and high-resolution transmission electron microscopy. We show that the energetically strongest attachment of the radicals occurs along the armchair direction in graphene to carbon atoms residing in different graphene sub-lattices. The radicals tend to assume vertical position on graphene substrate irrespective of direction of the bonding and the initial configuration. The “standing up” molecules, covalently anchored to graphene, exhibit two types of oscillatory motion – bending and twisting – caused by the presence of acoustic phonons in graphene and dispersion attraction to the substrate. The theoretically derived mechanisms are confirmed by near atomic resolution imaging of individual perchlorocoronene (C24Cl12) molecules on graphene. Our results facilitate the understanding of controlled functionalization of graphene employing electron irradiation as well as mechanisms of attachment of impurities via the processing of graphene nanoelectronic devices by electron beam lithography.

 Please wait while we load your content...
Please wait while we load your content...