Nonresonant chemical mechanism in surface-enhanced Raman scattering of pyridine on M@Au12 clusters†
Abstract
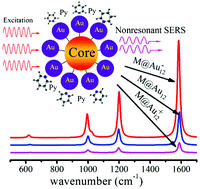
By employing density functional theory (DFT), this study presents a detailed analysis of nonresonant surface-enhanced Raman scattering (SERS) of pyridine on M@Au12 (M = V−, Nb−, Ta−, Cr, Mo, W, Mn+, Tc+, and Re+)-the stable 13-atom neutral and charged gold buckyball clusters. Changing the core atom in M@Au12 enabled us to modulate the direct chemical interactions between pyridine and the metal cluster. The results of our calculations indicate that the ground-state chemical enhancement does not increase as the binding interaction strengthens or the transfer charge increases between pyridine and the cluster. Instead, the magnitude of the chemical enhancement is governed, to a large extent, by the charged properties of the metal clusters. Pyridine on M@Au12 anion clusters exhibits strong chemical enhancement of a factor of about 102, but the equivalent increase for pyridine adsorbed on M@Au12 neutral and cation clusters is no more than 10. Polarizability and deformation density analyses clearly show that compared with the neutral and cation clusters, the anion clusters have more delocalized electrons and occupy higher energy levels in the pyridine–metal complex. Accordingly, they produce larger polarizability, leading to a stronger nonresonant enhancement effect.


 Please wait while we load your content...
Please wait while we load your content...