The 3,4-dioxygenated 5-hydroxy-4-aryl-quinolin-2(1H)-one alkaloids. Results of 20 years of research, uncovering a new family of natural products†
Abstract
Covering: up to April 2016
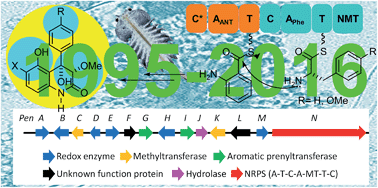
Aspergillus and Penicillium are fungal species known to produce a high diversity of secondary metabolites, many of them endowed with interesting bioactivity. The small but steadily growing family of the naturally occurring 5-hydroxy-4-aryl-quinolin-2(1H)-one alkaloids and closely related compounds, which represent the results of various research projects that spanned over 20 years and involved scientists from different continents, are covered here. Emphasis is placed on the isolation and chemical structures of the different compounds, together with their source microorganisms, environmental conditions, country or region of origin, and relevant biological activities. In addition, stereochemical aspects, as well as the proposed biosynthetic pathways for the different members, and the incipient synthetic efforts towards some of the compounds or their key intermediates, are discussed in detail.

 Please wait while we load your content...
Please wait while we load your content...