C,N-Chelated organotin(iv) azides: synthesis, structure and use within click chemistry†
Abstract
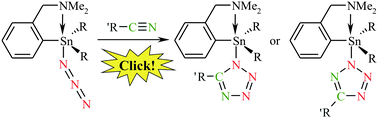
A set of tri- and diorganotin(IV) azides bearing 2-(N,N-dimethylaminomethyl)phenyl as a C,N-chelating ligand (LCN) has been prepared and structurally characterized. Triorganotin(IV) azides of the type LCNR2SnN3 (R = n-Bu (1) and Ph (2)) and (LCN)2(n-Bu)SnN3 are monomeric both in solution and in the solid state. The central tin atom in these species is five-coordinated with distorted trigonal bipyramidal geometry. Diorganotin(IV) azides of the type LCNRSn(N3)2 (R = n-Bu and Ph) are monomeric with trigonal bipyramidal geometry around the tin atom as well. Finally, (LCN)2Sn(N3)2 contains a six-coordinated tin atom with heavily distorted octahedral geometry due to the presence of two LCN units. The potential use of selected organotin(IV) azides 1 and 2 as useful building blocks within click chemistry was investigated. The reactions of 1 and 2 with various nitriles resulted in the formation of corresponding triorganotin(IV) tetrazolides (i.e. κ-N1: LCN(n-Bu)2Sn(5-MeCN4), LCNPh2Sn(5-MeCN4), LCN(n-Bu)2Sn(5-Me2NCH2CN4), LCNPh2Sn(5-Me2NCH2CN4); and κ-N2: LCN(n-Bu)2Sn(5-t-BuCN4), LCNPh2Sn(5-t-BuCN4), LCN(n-Bu)2Sn(5-PhCN4), LCNPh2Sn(5-PhCN4)). Similarly, the reaction of 1 and 2 with cyclooctyne provided corresponding C,N-chelated di-n-butyl/diphenyltin(IV) κ-N1 4,5,6,7,8,9-hexahydrocycloocta[d][1,2,3]triazol-1-ides. All azido complexes and products of the [3+2] cycloaddition reactions were characterized by the combination of elemental analysis, mass spectrometry, IR spectroscopy, multinuclear NMR spectroscopy and, in the case of crystalline materials, XRD analysis. In addition, DFT calculations were carried out within the click chemistry reactions in order to corroborate the preferred formation of the respective tetrazolide regioisomer.
- This article is part of the themed collection: Nitrogen Ligands

 Please wait while we load your content...
Please wait while we load your content...