Sulfated polyborate: a new and eco-friendly catalyst for one-pot multi-component synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones via Biginelli reaction†
Abstract
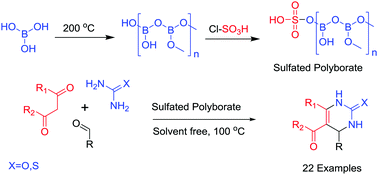
In this report, an efficient high-yield method for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones via a three-component Biginelli reaction with various aldehydes, β-ketoesters/β-diketones and urea/thiourea is described. The reaction was catalyzed by sulfated polyborate under solvent free conditions at 100 °C. The catalyst was prepared and used as a Brønsted as well as Lewis acid catalyst. The catalyst was prepared by dehydration of boric acid followed by sulfonation and was characterized by different analytical techniques. The key advantages of the present method are the ability to tolerate a variety of functional groups, high yields, short reaction times, solvent free conditions, easy work-up, and recyclability of the catalyst, thus providing economical as well as ecological advantages.


 Please wait while we load your content...
Please wait while we load your content...