Anhydrides from aldehydes or alcohols via oxidative cross-coupling†
Abstract
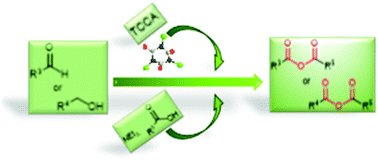
A novel type of metal-free oxidative cross-coupling for the synthesis of symmetrical and mixed anhydrides from aldehydes or benzylic alcohols has been developed. The aldehydes or alcohols were converted in situ into their corresponding acyl chlorides, which were then reacted with an array of carboxylic acids. The methodology has a general applicability, and was successfully employed to prepare either aromatic or aliphatic symmetrical anhydrides and mixed anhydrides, which are very unstable compounds.


 Please wait while we load your content...
Please wait while we load your content...