NMR detection of chirality and enantiopurity of amines by using benzene tricarboxamide-based hydrogelators as chiral solvating agents†
Abstract
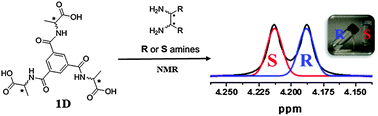
Enantiomeric excess of chiral compounds is a key parameter that can influence their activity or therapeutic action. Current approaches to the rapid measurement of enantiomeric excess using 1H NMR is based on the formation of diastereomeric complexes between chiral analytes and a chiral host, leading to two species with no symmetry relationship. Here, we demonstrate that a gelator host system can provide a means to elicit distinct chemical shifts in the 1H nuclear magnetic resonance (NMR) of a mixture of diamines or monoamines depending on the corresponding enantiomeric excess of the guest mixture of chiral amines. These gelator hosts provide a unique example of NMR based assessment of the chirality and enantiopurity of guest amines.

 Please wait while we load your content...
Please wait while we load your content...