Promotion of 1,3-dipolar cycloaddition between azides and β-enaminones by deep eutectic solvents†
Abstract
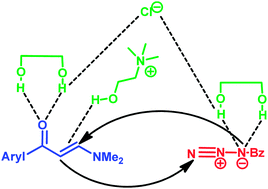
A simple procedure to obtain 4-acyl-1-substituted-1,2,3-triazoles, using a deep eutectic solvent (DES), ChCl and ethylene glycol at a 1 : 2 ratio, as the reaction medium is described. The products were obtained in high selectivity and good yields (70–84%). The advantages of the method include easy work-up, metal-free conditions, inexpensiveness, and the ability to be used four times without a loss in yield.

 Please wait while we load your content...
Please wait while we load your content...