Bargellini condensation of ninhydrin as a ketone and substituted anilines as nucleophiles†
Abstract
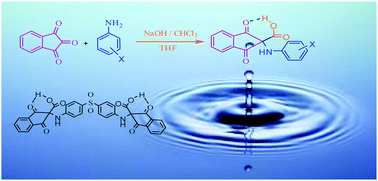
A series of novel α-amino acids based on ninhydrin were synthesized by using various substituted anilines as nucleophiles with ninhydrin in the presence of chloroform and NaOH as base. All the compounds were fully characterized. All reactions were completed under ambient reaction conditions and all products were obtained in high to excellent yields and with high purity.

 Please wait while we load your content...
Please wait while we load your content...