Impact of chabazite SSZ-13 textural properties and chemical composition on CO2 adsorption applications†
Abstract
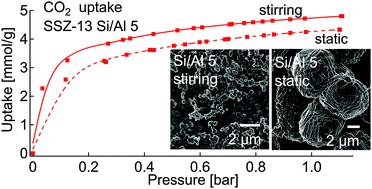
Chabazite SSZ-13 samples with varying silica content (Si/Al from 5 to ∼20) were synthesized under both stirring and static conditions to obtain materials with changing particle size and morphology and thoroughly analysed using various characterization techniques. The role of particle size and chemical compositions in CO2 and N2 adsorption measurements was investigated. The Si/Al ratio played a major role in CO2 adsorption; Al-rich SSZ-13 demonstrated a higher CO2 uptake than an Al-poor material. This was attributed to the high density of active charged species in the chabazite cage. The particle size also played a role in the sorption capacities; smaller particles, obtained under stirring conditions, showed enhanced CO2 uptake compared to larger particles of similar chemical composition. This was associated with a higher contribution of micropores containing active sites for CO2 adsorption.
- This article is part of the themed collection: The Creative World of Porous Materials


 Please wait while we load your content...
Please wait while we load your content...