Controlling the morphology of calcium sulfate hemihydrate using aluminum chloride as a habit modifier†
Abstract
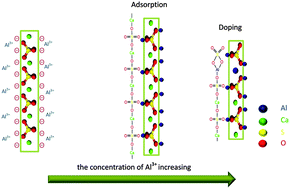
The morphology and aspect ratio of calcium sulfate hemihydrate (HH) are successfully controlled using AlCl3 as a morphology modifier in the hydrothermal synthesis process. As the concentration of AlCl3 is increased from 0 to 7.5 × 10−2 mol L−1, the crystal length decreases from 130 μm to 0.1–0.3 μm and the corresponding aspect ratio declined dramatically from 150–240 to 1–2, and the crystal morphology gradually changes from whiskers to rods, and even irregular nanogranules. The preferential adsorption of Al3+ on the side facets of HH would lower the surface energy and inhibit the elongation along these facets. The doping of Al3+ in the HH would destroy the main chain of HH and inhibit the elongation along the [001] direction. The two facts contribute to the morphology control. This work exhibits an efficient method to control the morphology of HH over a large range of size by simply adjusting the concentration of AlCl3.

 Please wait while we load your content...
Please wait while we load your content...