Impact of water on the melting temperature of urea + choline chloride deep eutectic solvent
Abstract
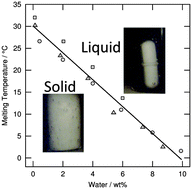
Deep eutectic mixtures are considered as promising green, cheap and easy-to-prepare solvents for applications in catalysis, extraction or material design. In the present work we focus on the urea:choline chloride system. Our aim was to establish its phase diagram and to quantify the impact of naturally present water on the melting temperature for this highly hygroscopic system. The phase diagram of dried urea:choline chloride was established using three complementary apparatuses: a thermostated bath, an optical microscope and a differential scanning calorimeter. Due to limited thermal stability, only mixtures with urea mole fractions between 0.50 and 0.80 were studied. The eutectic point was found for a urea mole fraction of 0.67 at 25 °C and its enthalpy of melting is 93 J g−1. Water can be easily absorbed from the atmosphere, which decreases the melting temperature of eutectic compositions below room temperature. This is quantified in this paper by a systematic study of the melting temperature of a eutectic mixture containing different quantities of water up to 10 wt%. The presence of water should be taken into account for any physico-chemical characterization as well as for applications of this type of eutectic system.

 Please wait while we load your content...
Please wait while we load your content...