On the influence of coordinating solvents on the reduction of selenium for the phosphine-free synthesis of metal selenide nanoparticles†
Abstract
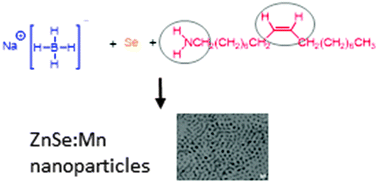
In this study, we report on the influence of organic solvents on the dissolution and reduction of elemental selenium for the phosphine-free synthesis of metal selenide nanoparticles. To this end, elemental selenium was reduced with sodium borohydride in the presence of a hydrophobic unsaturated alkylamine (oleylamine) and an alkene (1-octadecene) in different ratios. These varying conditions affect the optical properties of the prepared ZnSe:Mn nanoparticles (which was chosen as the test case) in a systematic way. The characterization with UV-vis absorption, photoluminescence measurements and nuclear magnetic resonance (NMR) shows the importance of the bifunctionality of oleylamine for the reduction and dissolution of elemental selenium for the scalable synthesis of highly crystalline nanoparticles. NMR data indicate that (in addition to the amine group) the double bond in the center of the molecule plays an important role as well. A proposed reaction pathway describes the specific actions of the amine group and the double bond in oleylamine for the synthesis of selenide nanoparticles. Consequently, oleylamine as solvent has a pronounced positive effect on the optical properties of the synthesized ZnSe:Mn nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...