A family of substituted hydrazonoisoxazolones with potential biological properties†
Abstract
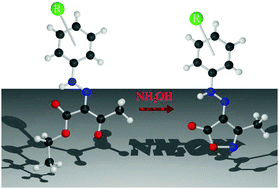
The synthesis, characterization and biological study of a new 3,4,5-trisubstituted isoxazolones have been reported, whereby a series of (Z)-3-methyl-4-(2-(R-phenyl)hydrazinylidene)isoxazol-5(4H)-ones were prepared by the reaction of a β-diketohydrazone with hydroxylammonium chloride. All the products were characterized using EA, UV-Vis, FT-IR, 1H-NMR, 13C-NMR spectroscopy and HMBC. The crystalline and molecular structures of three compounds were solved by X-ray diffraction methods. Density functional theory (DFT) and time-dependent DFT (TDDFT) calculations were performed to obtain a better explanation of the observed experimental behaviour of these newly synthetized compounds. Furthermore, the reports of cytotoxicity and an antiproliferative effect in human promyelocytic leukaemia cells, HL-60, was tested by the MTT reduction method, showing that most of the newly synthetized compounds had important antineoplastic activity. The most active isoxazolones were used in reverse transcription polymerase chain reaction (RT-PCR) experiments to determine the effect on the expression levels on mRNA encoding using the anti-apoptotic, Bcl 2, pro-apoptotic, Bax, and the proliferation inhibition, p21WAF-1, proteins. Therefore, it was possible to fully characterize the complete library of 15 isoxazolones and to show that most of them are antineoplastic trough an apoptotic pathway.

 Please wait while we load your content...
Please wait while we load your content...