Hierarchical growth of ZnFe2O4 for sensing applications†
Abstract
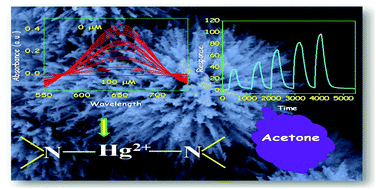
Mesoporous ZnFe2O4 nanoflowers (NFs) have been prepared using a modified hydrothermal (MHT) technique, developed in our laboratory. Urea has been brought in for hydrolysis of FeCl3 and ZnSO4 in solution to homogeneously precipitate ZnFe2O4. The precipitated product upon annealing at 450 °C results in mesoporous ZnFe2O4 NFs. Important physical methods have been used to characterize the NF material in the solid state. The growth mechanism of mesoporous NFs of evolution is confirmed by the adopted reaction strategy. The ZnFe2O4 NF finds application in peroxidase mimicking activity which in turn helps the selective naked eye detection of H2O2 and Hg2+ ions in solution. However, spectrophotometric detection limit goes down to 0.1 mM and 2.58 × 10−3 mM for H2O2 and Hg2+, respectively. To contemplate peroxidase like activity, colorless 3,3′,5,5′-tetramethylbenzidine (TMB) is employed which in turn oxidized to a blue solution by H2O2 in the presence of ZnFe2O4 rendering H2O2 sensing. It has been discovered that the blue color development is selectively held up by Hg2+ ion causing Hg2+ sensing possible. Judicious selection of Hg2+ ions once again indicates strong affinity of the nitrogen donor towards Hg2+. This makes Hg2+ sensing possible without the use of any noble metal. A strong and definite ‘–N–Hg–N’ binding interaction with nitrogen donors of the TMB substrate causes blue color bleaching. Herein we report the usefulness of an under-rated ZnFe2O4 nanoflower for the first time to detect Hg2+ spectrophotometrically and in a cost-effective way. On the other hand, a highly mesoporous nanoflower has been shown to be a selective sensor for acetone also. Based on the above reaction/interaction strategies it is expected that the as-synthesized ZnFe2O4 NFs would stand as cost effective sensor materials for biological applications and environmental remediation.

 Please wait while we load your content...
Please wait while we load your content...