Facile solvolysis of a surprisingly twisted tertiary amide†
Abstract
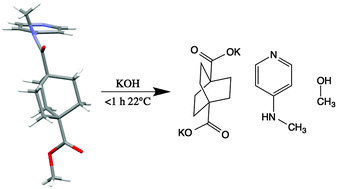
A bicyclo[2.2.2]octane derivative containing both a tertiary amide and a methyl ester (1) was shown crystallographically to adopt a conformation in which the amide is in the cis configuration, which is sterically disfavored, but electronically favored. The steric strain induces a significant torsion (15.9°) of the amide, thereby greatly increasing the solvolytic lability of the amide to the extent that we see competitive amide solvolysis in the presence of the normally more labile methyl ester also present in the molecule.

 Please wait while we load your content...
Please wait while we load your content...