Abstract
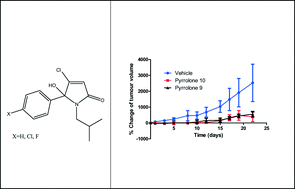
A new class of 5-arylated 5-hydroxypyrrolones was derived from mucochloric acid in 2 synthetic steps and the chemical structure was confirmed additionally by X-ray analysis. Using a radiolabelled binding assay, potent CCK1 selective ligands were identified (CCK1: 12 nM) and the antagonism was confirmed by using isolated tissue preparations. A series of isobutyl derivatives displayed unsurmountable CCK antagonistic properties and in vitro excellent inhibition of proliferation was obtained in cholecystokinin related cancer cell lines in the nanomolar range. Finally, using xenograft studies in nude mice, two selected pyrrolone derivatives, X = H and X = F a fluorinated analogue (PNB-028 ), showed a strong inhibition of tumour growth in a chemo-resistant colon cancer-(MAC 16) and a human pancreatic cell line (MIAPACA) at 50 mg kg−1 by oral administration.

 Please wait while we load your content...
Please wait while we load your content...